

West Valley City, Utah and Sydney, Australia — October 16, 2025
Clarity Pharmaceuticals (ASX: CU6), a clinical-stage radiopharmaceutical company based in Sydney, has signed a three-year copper-67 supply agreement with Nusano, Inc., the West Valley City physics company building one of the most advanced isotope production facilities in the world.
The deal gives Clarity a dependable U.S. source for copper-67, a promising therapeutic isotope used in next-generation cancer treatments, and marks another milestone for Nusano’s $300 million West Valley City campus.
When fully operational in mid-2026, Nusano’s 190,000-square-foot facility will anchor what the company calls a new era of domestic isotope production—one designed to stabilize supply chains that have long depended on aging nuclear reactors abroad.
“We are excited to continue growing our supply network for copper-67 in preparation for a registrational Phase III clinical trial,” said Dr. Alan Taylor, Executive Chairperson of Clarity. “By employing copper-67, we can avoid many of the drawbacks of other therapeutic isotopes. With Nusano’s alternative production methods, we are further differentiating our supply and look forward to having numerous pathways to producing large quantities of copper-67.”
Utah’s $300 Million Bet on Isotope Sovereignty
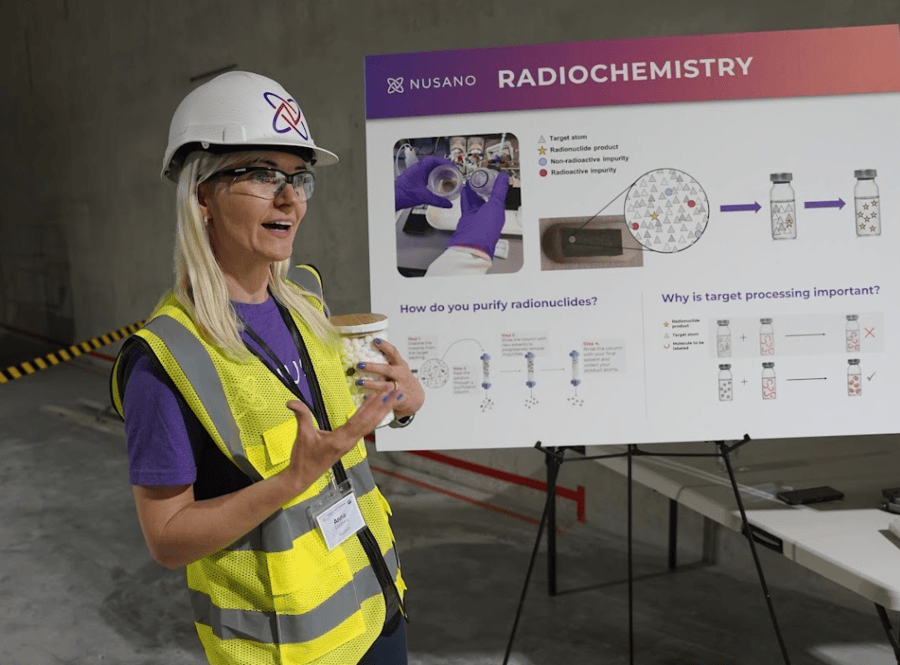
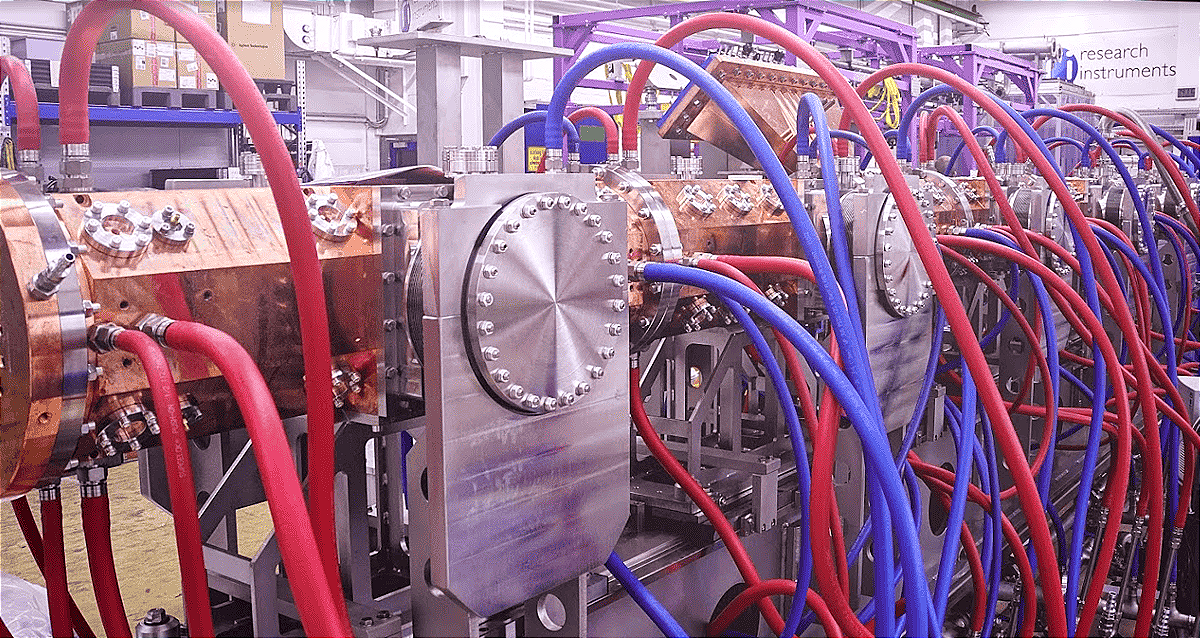
In TechBuzz’s earlier coverage, Nusano unveiled its $300 million investment in West Valley City—a sprawling complex built on 688 earthquake-resilient concrete pillars and engineered to operate in a 7.0-magnitude quake. The facility is powered by Nusano’s proprietary ion-beam accelerator technology, which can produce more than 25 medical and industrial isotopes simultaneously.
Unlike traditional isotope production, which relies on a small fleet of reactors concentrated overseas, Nusano’s accelerator-based method depends only on electricity and readily available materials. This allows for safer, cleaner, and more scalable production—an essential shift for the fast-growing radiopharmaceutical sector.
Nusano’s CEO Chris Lowe described the company’s platform as a “once-in-a-generation infrastructure” investment for the United States.

“We are commercializing a breakthrough radioisotope production platform in 2025 capable of producing more than 25 radioisotopes for life science applications,” Lowe said. “Clarity is in a unique position with their copper-67 program, being the only radiopharmaceutical company with ongoing clinical trials utilizing this important isotope.”
Clarity’s Copper-67 Program: A Fast-Tracked Contender
Clarity is developing a pipeline of Targeted Copper Theranostics, a new class of radiopharmaceuticals that combine diagnostic imaging and targeted therapy in a single isotope pair. The company’s lead product, 67Cu-SAR-bisPSMA, targets metastatic prostate cancer and has already received Fast Track Designation from the U.S. FDA.
The company is now preparing for a Phase III clinical trial, supported by encouraging early-stage data from its SECuRE study. Reliable access to therapeutic copper-67 will be critical for scaling clinical and eventual commercial supply.
The agreement with Nusano adds another American supplier to Clarity’s growing network, which includes NorthStar Medical Radioisotopes and the Idaho Accelerator Center at Idaho State University.
The Supply Chain Advantage
Nusano’s platform gives Clarity what has been missing in the isotope market: supply stability. Historically, isotopes like lutetium-177 have faced chronic shortages due to reliance on an aging global reactor fleet. Reactor shutdowns or maintenance cycles have caused widespread delays in radiopharmaceutical trials and patient treatments.
Copper-67 offers a way out of that bottleneck. Its production through accelerator systems, rather than reactors, means it can scale faster and with less geopolitical risk. Nusano’s investment in its own stable isotope enrichment line for copper-67 starting materials further reduces dependence on imported feedstock, particularly from Russia—a critical consideration as global supply chains tighten.
Utah’s Emerging Nuclear-Tech Cluster
For Utah, the Nusano-Clarity agreement is more than a business deal; it’s a validation of the state’s emerging role in advanced nuclear and medical technology. Nusano’s campus is projected to create hundreds of high-skilled jobs and establish West Valley City as a nucleus for the production of medical isotopes, rare earths, and materials for nuclear energy.
As TechBuzz previously reported, Nusano’s ambitions extend beyond medicine. The company’s accelerator technologies are also being developed for rare earth element separation, advanced materials research, and clean energy production, including components for high-assay low-enriched uranium (HALEU) fuel cycles.
By weaving together healthcare, national security, and clean technology, Nusano is positioning itself as a cornerstone of America’s next-generation isotope infrastructure.
Looking Ahead
The copper-67 supply agreement between Clarity and Nusano is effective immediately and includes automatic renewal provisions beyond its initial three-year term. Production is expected to begin in mid-2026.
While Clarity’s 67Cu-SAR-bisPSMA remains an unregistered product awaiting full regulatory review, the agreement marks a clear signal of commercial readiness—and of confidence in Nusano’s capabilities.
As Utah’s isotope hub takes shape, deals like this one move the conversation from vision to execution, making West Valley City an increasingly visible player in the global race to modernize cancer treatment and secure domestic isotope supply.
To learn more, visit nusano.com and claritypharmaceuticals.com.

