

West Valley City, Utah and Berlin, Germany — October 15, 2025
The radiopharmaceutical sector, long constrained by scarce medical isotopes, is gaining a rare infusion of strategic certainty. Nusano, a privately held physics company developing accelerator-based radioisotope production, and Ariceum Therapeutics, a clinical-stage oncology firm based in Berlin, have signed a multi-isotope supply agreement that gives Ariceum access to actinium-225 (Ac-225) and lutetium-177 (Lu-177). These isotopes are essential to Ariceum’s pipeline of targeted radiotherapeutics, including its lead candidate, 225Ac‑SSO110, currently in Phase 1/2 trials for extensive-stage small cell lung cancer (ES‑SCLC) and Merkel cell carcinoma (MCC).
The deal is emblematic of a growing trend: radiopharma developers securing dedicated supply arrangements to mitigate a chronic bottleneck. “Reliable access to actinium-225 ensures we can deliver on our commitment to developing transformative therapies,” said Manuel Sturzbecher-Höhne, Ariceum’s chief technology officer.

Ariceum’s Pipeline: High-Precision Radiotherapy
Headquartered in Berlin, Germany, and operating across Germany, Switzerland, Australia, the United Kingdom, and the United States, Ariceum Therapeutics is a clinical-stage oncology company dedicated to redefining the future of care through targeted radiotherapeutics for patients with aggressive and hard-to-treat cancers. The company’s lead program, 225Ac-SSO110, a novel antagonist of the somatostatin type 2 receptor (SSTR2) with best-in-class potential, is currently being investigated in the Phase 1/2 SANTANA-225 study as the first maintenance radiotherapy for extensive stage small cell lung cancer (ES-SCLC) and Merkel Cell Carcinoma (MCC) — two diseases with limited options and poor prognosis. Ariceum is also developing a clinical product candidate ATT001, a novel radiolabeled I-123 PARP inhibitor designed to deliver subcellular precision radiotherapy to aggressive solid tumors.
Unlike conventional chemotherapies, which affect both healthy and cancerous cells, targeted radiotherapeutics deliver radiation specifically to tumor sites. Early clinical data suggest that alpha-emitters like Ac-225, which emit highly destructive radiation over a very short range, may be particularly effective against aggressive and underserved cancers such as ES‑SCLC and MCC.
In addition, Ariceum is developing ATT001, a radiolabeled I-123 PARP inhibitor designed to deliver precision radiotherapy at the subcellular level. While still in preclinical development, ATT001 represents the company’s broader strategy: leveraging isotopes to create highly targeted therapies that minimize collateral damage to healthy tissue. Both programs rely on consistent access to high-purity isotopes, without which dose scheduling, clinical trial progression, and eventual commercialization become severely constrained.
Reliable isotope access fundamentally shapes therapeutic strategy. Drug developers cannot test multiple dosing regimens, expand patient cohorts, or explore combination therapies without sufficient isotopes. Delays or shortages directly impact trial timelines, regulatory submissions, and investor confidence. For Ariceum, securing a dedicated supply from Nusano effectively removes a major structural bottleneck, allowing the company to plan trials and scale production with greater certainty.
A Persistent Supply Challenge
Actinium-225 and lutetium-177 are critical for targeted radiotherapies, but their production has historically been constrained. Traditional sources rely on aging nuclear reactors or cyclotrons, often with complex international supply chains. Shortages and delays have repeatedly forced drug developers to postpone clinical trials or scale back production.
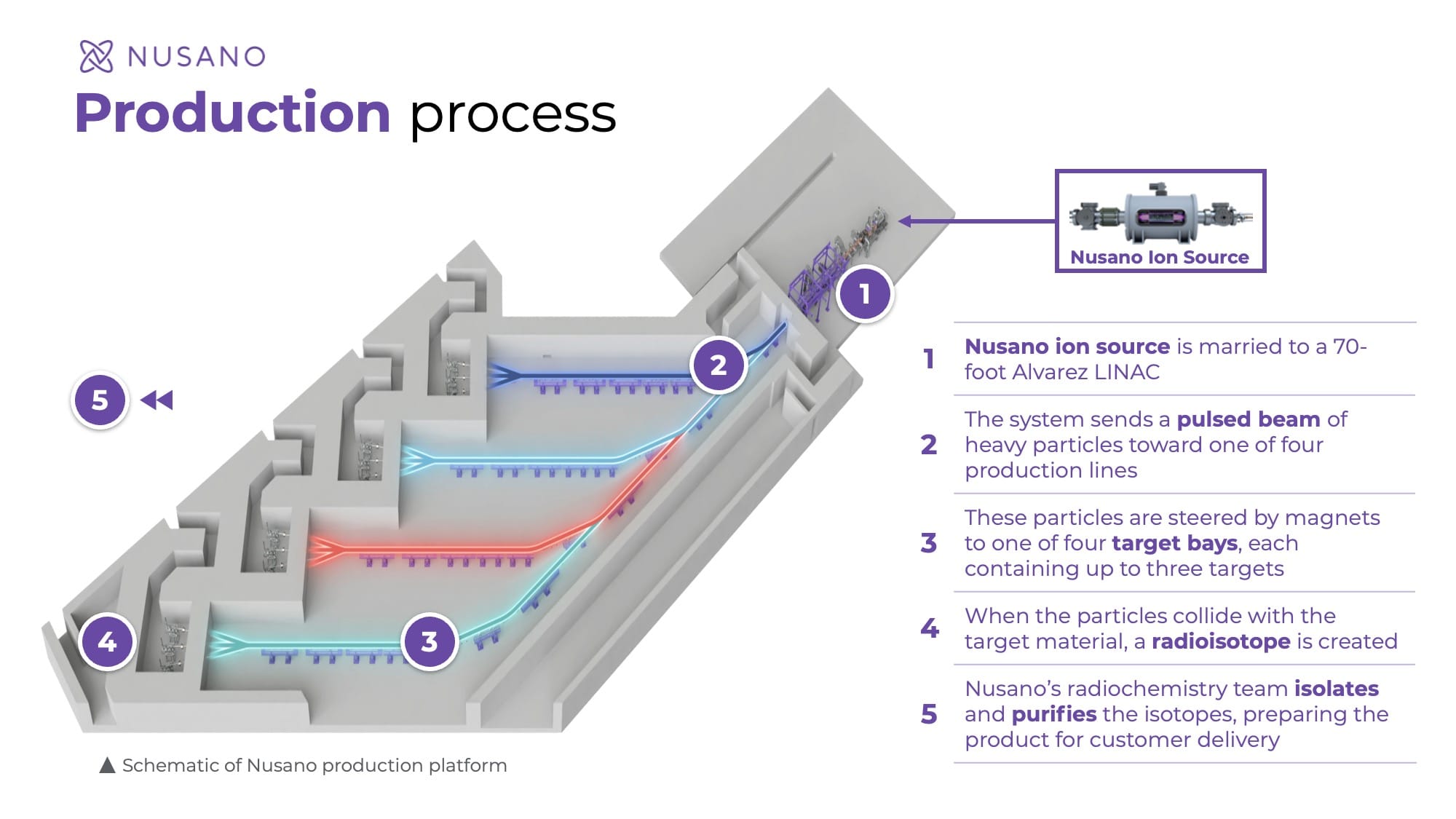
Nusano positions itself as a market solution. Its accelerator-based platform is designed to produce multiple isotopes simultaneously and on a scalable basis, claims the company, reducing dependency on a limited number of reactors and suppliers. Chris Lowe, Nusano’s CEO, emphasizes that the technology is meant to stabilize supply chains while supporting early-stage development through to commercial launch.
This is a potentially pivotal advantage. For companies like Ariceum, securing isotopes in sufficient volume is a prerequisite to scaling clinical programs, meeting regulatory timelines, and attracting investors. In a market where a single isotope shortage can stall a program, a dedicated supplier is a form of strategic insurance.

Strategic Implications for Investors and Developers
The agreement has significance beyond the immediate Nusano-Ariceum partnership. The ability of Nusano and Ariceum to reliably supply alpha-emitters like Ac-225 could shape which therapeutics reach the market first.
From an investment perspective, this deal signals two key points: first, that Nusano has reached a maturity level capable of entering commercial agreements; second, that the market values control over supply chains as highly as the therapies themselves. While venture capital has historically flowed to drug developers, the emergence of infrastructure plays in radiopharma — companies producing isotopes, enrichment technologies, and separation systems — is creating a parallel market dynamic.
Implications for Radiopharmaceuticals Market
The deal underscores a fundamental shift in radiopharmaceuticals: access to isotopes is as critical as the drug molecule itself. Nusano is positioning itself as a serious infrastructure provider, analogous to cloud or semiconductor companies in their respective markets, that is supplying a critical upstream component without which downstream innovation cannot scale.
For Ariceum, the agreement reduces one key barrier to commercializing 225Ac‑SSO110 and other pipeline candidates. Reliable isotope access enables the company to pursue ambitious dosing schedules, explore combination therapies, and expand clinical trial enrollment without supply constraints. For investors, it highlights the need to assess not only the science behind radiotherapeutics but also the stability of the supply chains on which these therapies depend.
In an industry still defining itself, the Nusano–Ariceum pact is a bellwether. It illustrates how strategic control over inputs — isotopes, enrichment technologies, and production infrastructure — may determine who succeeds in bringing next-generation radiopharmaceuticals to market. Execution will be the ultimate test: delivering on promise, at scale, on time, and within regulatory standards.
To learn more, visit Nusano.com and ariceum-therapeutics.com.


