

American Fork, Utah — January 20, 2026
PhotoPharmics, Inc., a clinical-stage medical device company based in American Fork, Utah, has published peer-reviewed results from its Phase 2 clinical trial of Celeste®, an investigational photo-neuromodulation device for people living with Parkinson’s disease.
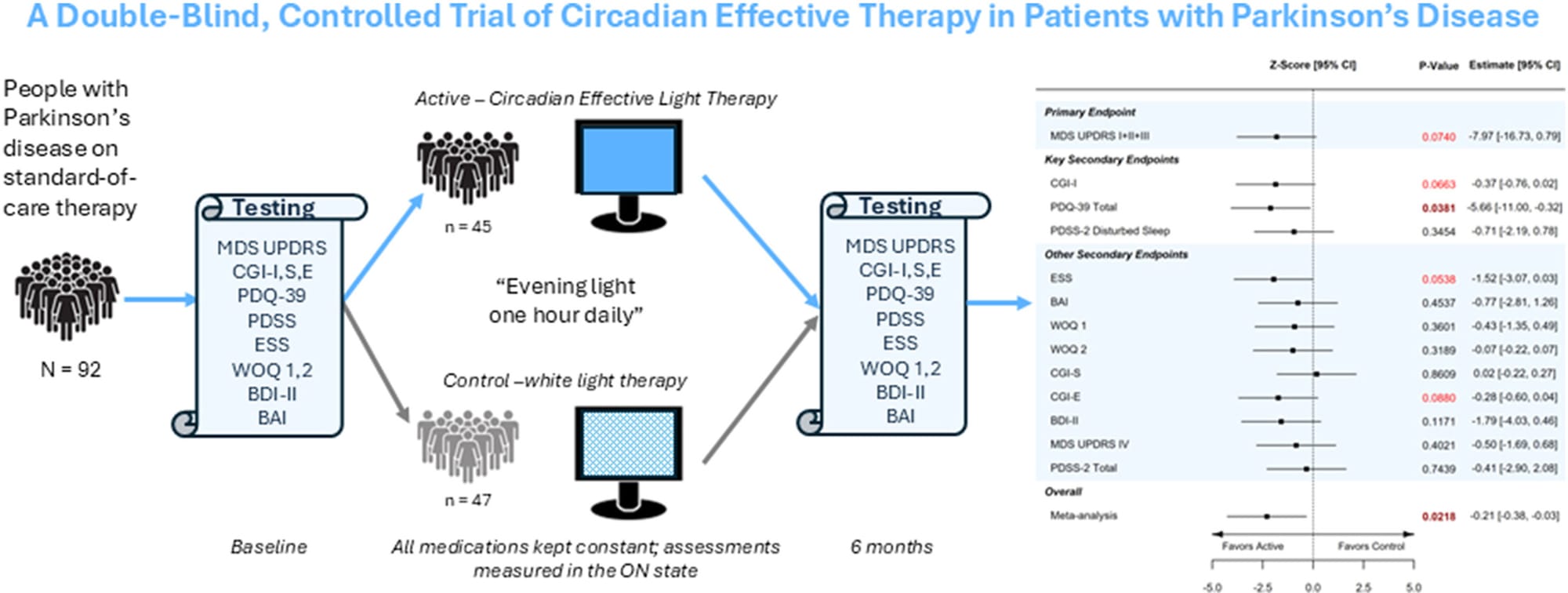
The randomized, double-blind, controlled study appears in Neurotherapeutics and evaluated daily use of Celeste over six months in patients receiving standard Parkinson’s therapies. Celeste is designated a Breakthrough Device by the U.S. Food and Drug Administration.
The Phase 2 trial did not meet its primary endpoint—change in the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) total score—at statistical significance (p = 0.074). However, researchers observed an eight-point between-group difference at six months and statistically significant improvements on select secondary measures, including quality of life as measured by the Parkinson’s Disease Questionnaire-39 (PDQ-39) and combined non-motor and daily-living components of the MDS-UPDRS.
Importantly, the device was well tolerated, with no serious adverse events reported during the study period.
According to the company, the Phase 2 results directly informed the design and powering of its ongoing Phase 3/Pivotal trial, which is fully enrolled and expected to complete in the second quarter of 2026.
“The Phase 2 study delivered exactly what it was supposed to—clear learning that helped us refine our pivotal trial,” said Dan Adams, PhotoPharmics’ chief science officer.
The study evaluated a proprietary spectral band of light designed to engage circadian pathways, a biological system increasingly linked to both motor and non-motor symptoms of Parkinson’s disease. The authors concluded that the findings support continued investigation of daily photo-neuromodulation in larger, double-blind trials.

“Publishing these data in a peer-reviewed journal reflects our commitment to scientific rigor and transparency,” said CEO Kent Savage. “The Phase 2 trial was a critical step in advancing Celeste toward a definitive evaluation.”
PhotoPharmics completed enrollment for its Phase 3/Pivotal trial in October 2025. Data collection is expected to continue through April 2026, with results anticipated later in the year.
The full study is available through Neurotherapeutics, the journal of the American Society for Experimental Neurotherapeutics (ASENT).
PhotoPharmics is a privately held, clinical-stage medical device company developing next-generation therapies for neurodegenerative disorders using specialized light. Its founders previously pioneered light therapy for seasonal affective disorder, sleep disorders, and depression—technology later acquired by Philips-Respironics. With Celeste, PhotoPharmics is advancing the science of phototherapy to address Parkinson’s disease and beyond. The company raised a $6 million Series B extension round in April 2025 to accelerate trials and commercialization.
Learn more at photopharmics.com.

